Biologics are changing the way we treat disease, affecting where companies invest, and, most importantly, impacting patients’ lives. Last year, nearly half of the 37 new drugs approved by the U.S. Center for Drug Evaluation and Research (CDER) were biologics, while its partner agency—the U.S. Center for Biologics Evaluation and Research (CBER)—approved a dozen additional products. These approvals span many modalities and cover a diverse range of conditions, including many rare and hard-to-treat diseases. In 2022, we saw:
5 protein drugs (for indications including diabetes, kidney and liver disease, lipid-buildup disease, and skin conditions)
11 antibodies (for indications including multiple sclerosis, lymphoma, diabetes, cancer, psoriasis, eye disease, and blood disorders)
1 antibody-drug conjugate (for ovarian cancer)
1 small interfering RNA therapeutic (for polyneuropathy)
12 blood products, gene therapies, and vaccines (for conditions such as cancer, blood disease, neurologic dysfunction, and infection)
Biology Labs Embrace Change
The huge strides made in biologics research & development (R&D) laboratories around the world is nothing short of incredible; but it’s certainly not a miracle. Rather, it is thanks to years of work by those who uncovered disease processes, created software to make sense of sequence, structure and experimental data, and embraced new ways of working across traditional boundaries. The willingness of biology labs to evolve has been essential to biologics’ incredible rise. For many labs, advancing biologics discovery has meant adopting new technology, including:
Sequencing platforms, image detectors, and molecular techniques
Specialized bioinformatics, proteomics, and molecular biology tools to scrutinize data and characterize modifications
Cloud-computing to analyze huge volumes of data, like high-throughput sequence data
The next great difference maker? Getting those software tools to work seamlessly, so the data can flow smoothly through the entire discovery workflow.
The Next Great Change: Uniting Tools and Data
Biologics R&D can be a tangle to navigate: the complexity and scale of the biological systems, the analysis workflows, the data compute and storage requirements, all the way down to the manufacturing and regulatory requirements. These produce an incredible amount of complex data that researchers need to sift through to make the right decisions that lead to discoveries.
Rich data visualization is a critical part of the R&D process. And, certainly, visualization of a wide array of data types can help researchers better understand and validate their work. But biologics teams don’t want to see their data in disconnected silos. They need to access and interrogate how the data relate to each other at any given moment throughout the discovery process. They want to use it—quickly and collaboratively—to propel their research forward. What makes a difference for biologics R&D teams will be closing the gaps on software tools and data to accelerate innovation and make better research decisions, all while stretching research dollars.
Discovery Through Tightly Integrated Software
Biologics R&D teams can benefit immensely when all their research tools are seamlessly connected. While it’s useful to select test results and visualize the associated data at once, it’s a gamechanger to move one step farther and allow researchers to quickly branch their research questions, register and track the process changes, quickly order or perform new tests or analyses—all from a central hub. Think of it like “Google maps” for science, helping researchers orient themselves, see where they have been, where they want to go, and what’s the best way to get there. Dotmatics is helping make this a reality by integrating software tools where it matters most—like SnapGene, Geneious Prime and Geneious Biologics, and Protein Metrics — on a powerful and flexible scientific R&D platform that features experiment and data management capabilities, inventory and registration systems, and collaboration and reporting tools.
FAIR Data is a Discovery Accelerator
On top of integrating R&D instruments and software, the data these instruments produce must be linked to get the most value out of that data. Creating a centralized data repository can be a huge undertaking. There are numerous considerations, including standardizing data formats, managing copious amounts of data, establishing permission controls for internal users and external partners, and facilitating backend data exchange. ALL data within a central depository must be findable, accessible, interoperable, and reusable, or “FAIR.”
Dotmatics helps biologics teams work toward this vision by providing an enterprise R&D data platform and scientifically-aware data management tools. Together, these solutions help biologics teams transform their collective R&D data into knowledge and make sure discoveries aren’t missed. With them, users can search huge volumes of data, drill down into specific results, select and collate data sets to explore, and directly perform analyses using specialty tools like the GraphPad Prism scientific graphing and statistical analysis program.
Unified Data Flow Example Scenarios
Let’s look at a few scenarios where unified tools and data can help propel biologics R&D.
Onboarding and Empowering New Antibody Team Members
Imagine a new researcher joins an antibody discovery team and needs insight into previous efforts. In the past, this might have been impossible due to poor record keeping and brain drain. But if the team’s work has been detailed and preserved in a centralized FAIR data repository, the new member can access all historical R&D data. They can see what work has been done, when, how, and by whom. For example, they can quickly search for and explore registered antibodies, see all associated data like associated clones or expression vectors, and view production-related data (such expression and purification information) and assay results.
Next, they might want to narrow down which antibody candidates to pursue by pulling in assay data to “lay over” their sequence data and annotations (like amino acid liabilities) and then performing alignments. With these data and tools at their fingertips, the new team member can quickly get up to speed, avoid redundant efforts, build off existing knowledge, and focus on the best antibody candidates. This kind of access to the data also lets the new researcher re-analyze data as new insights or data are found.
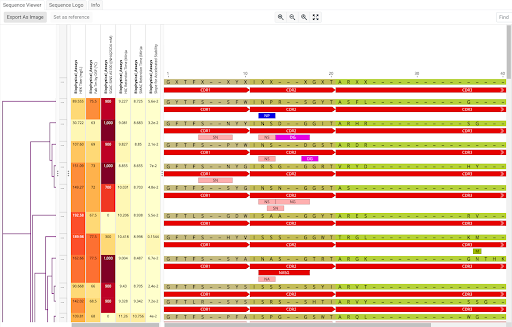
Antibody sequences annotated and visualized with CDR regions, sequence liabilities, assay results, and cladogram using the Geneious Biologics antibody sequence discovery platform.
Making Data-Driven Biologics Program Decisions
Another situation to consider is a biologics program director who needs to decide which disease target to pursue next. This typically involves an incredible amount of legwork to manually collect, process, and collate all the data needed to make good decisions, which is oftentimes done in an external program that traps the results forever. If that director could instead view relevant data in easy-to-interpret reports and dashboards, they could more quickly uncover the most promising program paths. Imagine, for example, the time that could be saved and knowledge gained if it were easy to collate data across assay experiments, perform bulk analyses like multiple curve calculations, and view results in publication-quality graphs.
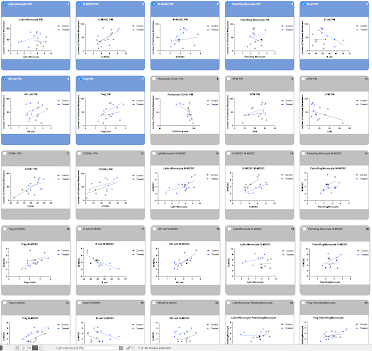
Program decisions are made easier with tools to graph and compare statistical analyses performed across multiple datasets at once.
Dotmatics Biology Solution
A centralized research platform delivers quick access to both the software tools and data that teams need to accelerate biologics discovery. With everyone from lab techs to antibody scientists to program directors united on the same platform, teams can easily:
Flow data between their specialty research tools
Build off each other’s knowledge
Streamline workflows and lab requests
Make data-driven decisions at every step
The Dotmatics Biology Solution supports drug discovery across a wide range of biologic therapeutic modalities by uniting the software tools research teams need for cloning, vector design, protein production, and assay screening, with the tools they need to visualize and analyze the resulting data and make well-informed decisions.
Learn more about the Dotmatics Biology Solution.
References
Halford, B. 37 new drugs achieved FDA approval in 2022. Chemical & Engineering News. January 21, 2023.
U.S. Food & Drug Administration. Center for Drug Evaluation and Research. Advancing Health Through Innovation: New Drug Therapy Approval 2022. (Accessed February 2, 2023).
U.S. Food & Drug Administration. Center for Biologics Evaluation and Research. 2022 Biological License Application Approvals. (Accessed February 2, 2023).