For more than two decades, researchers have leveraged our immune system to treat all kinds of diseases. It is really the only way personalized medicine can become a reality. As the complexities of the immune system are unraveled, researchers push the boundaries of possibility, designing new antibody types to treat the most complex and treatment-resistant diseases. But new antibody designs, like bispecifics, give rise to new challenges; researchers need the right molecular tools to succeed in the competitive and complex immunotherapy market, which is projected to reach tens of billions in the coming years.
What Are Antibody Therapeutics?
Antibody therapeutics are biologic drugs based on the body’s natural antibody proteins, which protect the body by recognizing and initiating an attack on foreign substances (antigens) or pathogens like viruses, bacteria, allergens, or cancer cells. As shown in Table 1, there are five different classes of antibodies, which are categorized based on their role and location in the body; nearly 75% of the antibodies floating around in our blood stream are immunoglobulin G (IgG) antibodies. IgG antibodies are produced by white blood cells as a natural part of the immune response, and they can also be designed in the lab for therapeutic or diagnostic use.
5 Antibody Classes
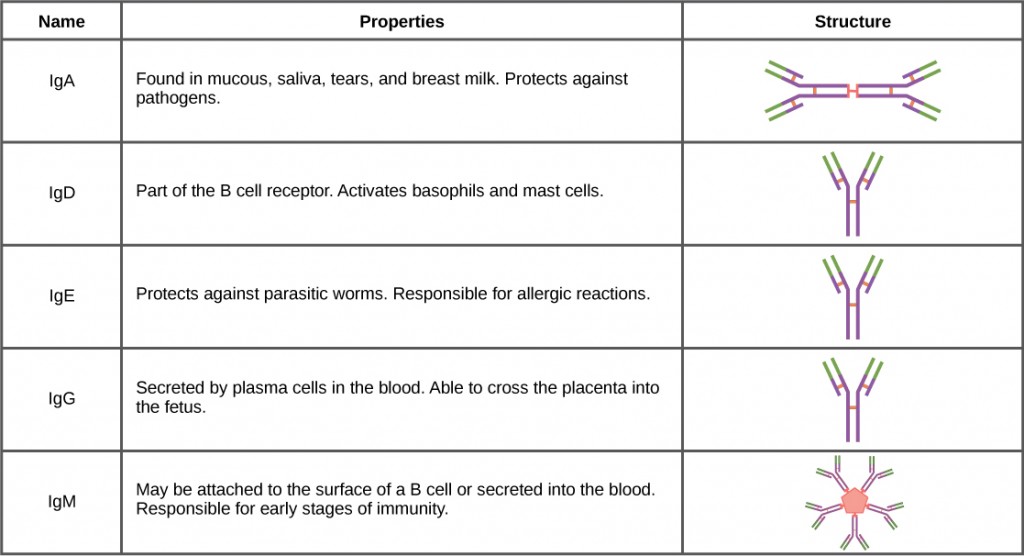
Table 1 (Source: https://opentextbc.ca/biology/chapter/23-3-antibodies/)
Natural vs Bispecific Monoclonal Antibodies
IgG and IgG-like antibodies are the main focus of biologics research groups and are made of symmetrical heavy and light protein chains. As shown in Figure 1, they are shaped like the letter Y, where the base (or constant Fc region) interacts with immune-system components and the two arms (or variable Fab region) recognize and interact with an antigen. On naturally occurring IgG antibodies, both ends of the arms of the “Y” structure bind the same target antigen.
IgG Antibody Structure
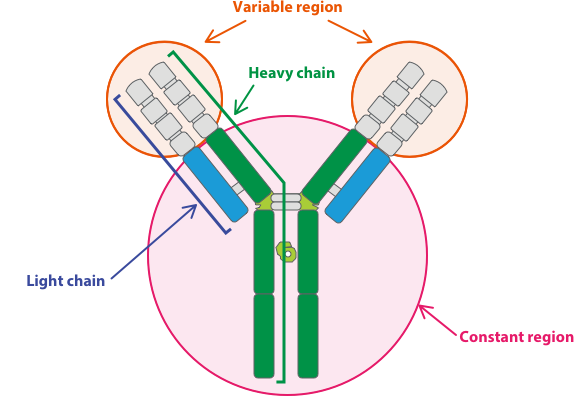
Figure 1 (Source: https://kkna.kyowakirin.com/what-we-do/research/)
Therapeutic monoclonal antibodies (mAbs) are produced using molecular cloning techniques that follow this structure; they are called ‘monospecific’ because they have just one target for both arms of the structure (at once).
Bispecific Antibodies
As shown in Figure 2, ‘bispecific’ antibodies (BsMabs), on the other hand, are built in such a way as to bind two distinct targets—one for each end of the arm; this might be two completely different antigens or two different binding sites (epitopes) on the same antigen.
Therapeutic Bispecific Antibody Format
Figure 2
Benefits of Bispecifics for Complex Disease
Why is pursuing more than one target important? Because complex diseases are hard to treat and gone are the days of one gene/one disease. A bsMab can target two disease mechanisms with one molecule. A singular approach may not suffice for conditions such as solid-tumor cancers, lymphoma, autoimmune diseases, or blood disorders. These conditions are often influenced by multiple factors over time and more than one angle of attack is often needed, such as when:
Multiple signaling pathways or mediators are implicated in the disease
A new pathway opens up after a primary pathway is blocked, leading to resistance or rebound
Immune inhibitors arise upon treatment and need to be blocked
An antibody needs assistance finding where it needs to be
The body’s own killer cells need to be recruited to completely destroy the pathogen
BsMabs may also help reduce toxicity issues and drug-drug interactions by achieving improved and synergistic effects without increasing dosage or adding drugs.
Bispecific Development
Designing bsMabs means combining different antibody building blocks to form an ideal structure with distinct binding properties. There are numerous design platforms for doing this and the potential resultant bispecific formats are plentiful and vary greatly in terms of their structure, affinity, mechanism of action, and pharmacokinetics.
The earliest method available to create bispecifics was the quadroma approach of fusing together two cell lines. This results in hybrid antibody products that mimic a natural IgG antibody structure, which is thought to have pharmacokinetic and activity benefits, including long half-lives. However, it is difficult to get the different heavy and light chains of the antibodies to fit together as intended and ineffective combinations are common, as shown in Figure 3. Various chain-match-improvement methodologies have been developed, starting with knobs-into-holes technology where intentional structural mutations are made to guide proper chain association. In addition to matching issues, engineering what are essentially “artificial” antibodies can also cause immunogenicity issues; as such, structural, modeling, assay, and testing data must be considered together to reduce the likelihood of a bispecific creating an unwarranted immune reaction.
Assembly Mispairings in Bispecific Antibody Development
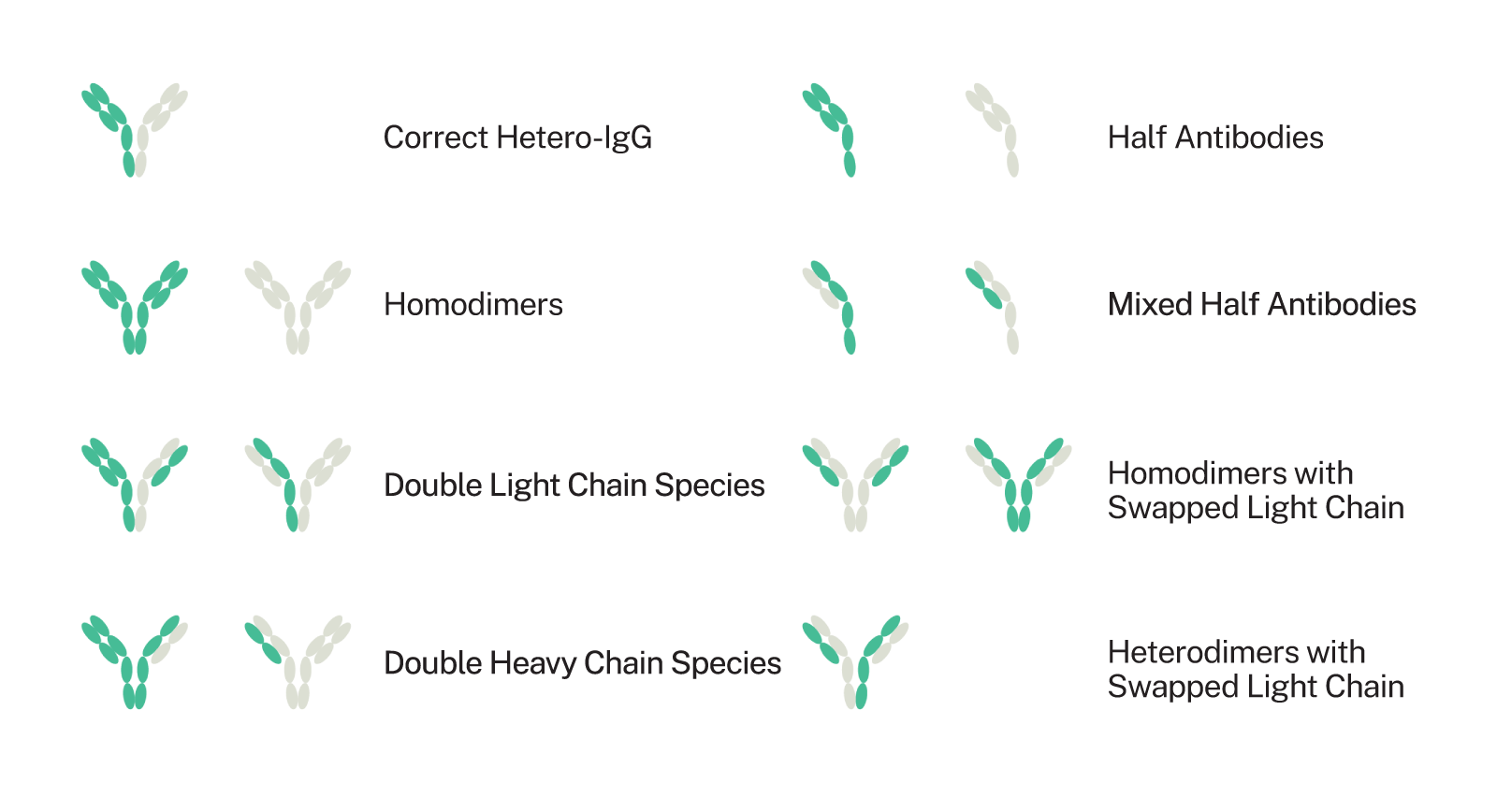
Figure 3
Other engineering technologies, including chemical conjugation with cross-linkers and recombinant DNA methods, are now also being used to create non-IgG-like fragmented structures that lack the antibody base. These products vary in their size, shape, and mechanism of action. While immunogenicity may be reduced because of their truncated format, the body tends to eliminate them quickly, which in turn requires a more continuous dosing schedule.
Today, there are hundreds of bispecific antibody formats, which are split into two main categories: 1) asymmetric, 2) symmetric. To date, there are only nine bispecific antibody therapeutics that have been approved by the Food and Drug Administration (FDA). All of them target cancer.
New Solutions for Bispecific and Multispecific Research
Developing BsMabs and multispecific antibodies demands new combinations of antibody parts that are as diverse and ingenious as our immune systems. This can challenge current research processes. Mass spectrometry (MS) is a mainstay for quantifying and characterizing antibodies. However, the traditional algorithms employed for deconvoluting MS data—so called “maximum entropy algorithms”—perform poorly when employed for novel molecular formats. They introduce artifacts that experts are required to manually sift through. Such bottlenecks hamper automation efforts in high throughput environments, as well as risk inconsistencies in data processing.
Protein Metrics by Dotmatics has developed a fundamentally different, patented approach. It leverages a parsimonious deconvolution algorithm which has two major advantages. Firstly, it is not burdened with the artifact peaks and can thus run unsupervised. Secondly, it is capable of treating data over a wider mass range, which makes it the ideal solution for intact mass analysis of new modalities like bsAbs.
The vendor-neutral solution enables users to go from raw data to report by employing pre-built workflows. These workflows can be customized, shared and scaled up for specific use cases, such as high-throughput exploration of different chain combinations.
Additional Antibody Solutions
In addition to this capability for robust, reproducible antibody characterization, Dotmatics offers a comprehensive collection of antibody discovery solutions to help teams:
capture all combinatorial protein data with fidelity
couple raw data with assay data
characterize and build massive protein-part libraries
characterize and screen millions of antibody sequences
make the most of time and budget by tracking all research efforts and easily discerning what worked and what didn’t
Learn More About Mass Spectrometry for Bispecifics
References
Global bispecific antibody market opportunity, drug sales, price & clinical trials insight 2028. Kuick Research. August 2022.
Kinthaert, L. What is an antibody? InformaConnect. September 2019.
Carrao, C. The emergence and benefits of bispecific antibodies. InformaConnect. July 2019.
Ma, J., Mo, Y., Tang, M., et. al. Bispecific antibodies: from research to clinical application. Front. Immunol., 12, 2021.
Wang, S., Chen, K., Lei, Qi., et. al. The state of the art of bispecific antibodies for treating human malignancies. EMBO Mol Med., 13, 2021

