Chemically-modified biologics, including various types of conjugates, modified peptides and novel nucleic acids, are an increasingly important part of many R&D programs. These novel entities can help reach new pharmacological space and overcome issues that conventional biologics often face in terms of dosing, targeting, pharmacokinetics and immunogenicity.
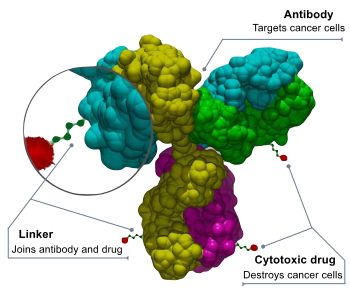
Two new treatments approved by the U.S. FDA in 2022 are great examples.
ELAHERE(TM) (irvetuximab - soravtansine-gynx) is an antibody-drug conjugate that provides a new targeted-treatment option for patients with treatment-resistant ovarian, fallopian tube, or primary peritoneal cancer.
AMVUTTRA(TM) (vutrisiran) is an RNA interference drug that treats neurological damage caused by hereditary transthyretin-mediated amyloidosis; though it targets the liver, it can be delivered subcutaneously just four times a year thanks to novel chemical-conjugate delivery technology that improves the drug’s metabolic stability, potency, and duration.
Despite their incredible potential, chemically-modified biologics create unique data management challenges for researchers pursuing them. What is the root cause of these challenges? The very nature of the entities themselves. As biologically-based molecules with artificial chemical modification, these novel entities require close collaboration between biology and chemistry teams who have traditionally worked in relative isolation from each other using different data types, workflows, and specialty R&D tools. The union of these two worlds is shaking up how cross-discipline teams innovate and increasing the demands placed upon the tools they use to capture, analyze, and share data.
Here we present the top five challenges that multidisciplinary teams face when collaborating on chemically-modified biologics R&D data.
1. Cross Domain Data Support
The biggest challenge for teams collaborating on chemically-modified biologics is finding an informatics platform that fully supports both chemical/small molecule structure and biological sequence data and metadata, and beyond that can make links between these entities. Without this support, biology and chemistry teams often continue using separate systems that don’t easily integrate. This not only slows collaborative innovation, but it can also lead to competing sources of truth, muddied entity descriptions and, inevitably, data silos are the price of admission and inevitable.
2. Data Quality and Loss
Capture of high-quality data is essential to interpreting results, progressing research, and applying advanced analytics like machine learning. When cross-discipline teams working on chemically-modified biologics manually move data between disconnected systems, or map and synchronize data transfers, they not only waste time, but also risk introducing errors or losing data. This can slow cycle times, increase programs costs, and unduly impact candidate selection.
3. Data Flexibility for User Needs
Chemistry and biology teams collaborating on chemically-modified biologics often want to see the same underlying data in different ways. Easy toggling between structure, sequence, and assay views is a must have. But beyond this surface-level usability feature, an informatics solution must also work behind the scenes to support the various R&D tools, processes, and data types used by cross-discipline researchers.
For example, teams may have molecular biologists who want to explore sequence variations, protein scientists who need to record purification protocols and analyze mass spectrometry results, medicinal chemists who want to enumerate compound libraries and analyze chemical properties, analytical scientists who want to determine drug-antibody ratios, screening scientists who need to collate and analyze assay results, etc.
While many solutions tout dual biology and chemistry support, few offer the platform breadth and configurability needed to let these diverse researchers capture, view, and analyze data in the ways most intuitive to them, while also feeding that data into a united source of truth.
4. Data Searchability and Traceability
While streaming, capturing, and parsing research data can be challenging in its own right, it’s often just the start of many teams’ struggles. Equally important is making the data searchable and traceable. This can be a tall order with the myriads of entities that are generated in chemically-modified biologics R&D. Teams are dealing with cross-discipline work, iterative research cycles, diverse research tools, and oftentimes large data sets. Manually collecting, collating, and relating all this data and metadata is impossible. Therefore, teams need their R&D platform to not only record experiment results, but also capture contextual data related to entity design, production and purification processes, experimentation steps, batch data, entity relationships, etc.
Biologists and chemists then need to be able to search across all the related data in the ways most intuitive to them; so, chemists may want to search by name, structure, or functional group and biologists by sequence motifs of similarity percentage. Any data point of interest should link all the way back to the exact test entity, letting researchers first see exactly what work was done, when, how, and by whom, and then letting them quickly use that data as a starting point to continue innovating within the same unified R&D platform; for example, chemists who find a structure of interest may want perform compound comparisons and request additional assays, while biologists may want to perform alignments, clustering, and filtering to explore mutations of interest.
A solution that unifies the data management and scientific analysis tools needed for chemically-modified biologics R&D is a gamechanger.
5. Data Exchange
Given the complexities of cross-discipline R&D, it’s no surprise that chemically-modified biologics R&D presents data-exchange challenges. We’ve already covered the challenges of equally accommodating the exchange of both biological sequences and chemical structure data. Beyond working across different scientific domains, teams also face challenges when working across different locations or partner organizations.
For example, in chemically-modified biologics research, activities such as compound synthesis or assay screening may be contracted to CROs. Or, a large pharma could sponsor key components of a discovery pipeline at a smaller biotech. Partnerships need an efficient way to receive requests and then return the results without relying on email attachments or manual upload of spreadsheets or documents. Without web-based, permission-controlled access to a unified R&D platform, cross-site teams will undoubtedly waste time, risk data error and loss, and compromise security when trying to exchange data.
Dotmatics Solutions for Chemically-Modified Biologics R&D
If you’re looking to create a complete informatics solution to support your research in chemically-modified biologics, look no further. Dotmatics can help you:
Unify all diverse biology and chemistry data on an enterprise data platform
Facilitate collaborative cross-discipline research
Simplify your informatics R&D infrastructure and reduce your lower total cost of ownership
Contact us today to learn more about our offerings, including:
An end-to-end R&D data platform with flexible scientific search
Personalizable ELNs with flexible support for biology and chemistry workflows
Complete biology and chemistry entity registration
Specialty tools for molecular biology, sequence analysis, proteomics, and compound design
Robust assay data management and inventory and lab-request management
United statistical analysis, visualization, and reporting for all R&D data
References
Halford, B. 37 new drugs achieved FDA approval in 2022. Chemical & Engineering News. January 21, 2023.
ELAHERETM (irvetuximab - soravtansine-gynx) product website. ImmunoGen, Inc. (Accessed February 14, 2023).
Amvuttra (vutrisiran) product website. Alnylam Pharmaceuticals. (Accessed February 14, 2023).