Managing the Madness:
Three Must-Dos for Tracking Multiformat Antibody R&D
Over the past decade, the concept of utilizing biologics to treat complex disease has undergone a significant transformation, emerging as a promising new reality. Monoclonal antibodies (mAbs) have been at the forefront of driving this progress. According to The Antibody Society, there are now nearly 200 antibody therapeutics either approved or under regulatory review. [1] However, the pursuit of biologic treatments is as intricate as the structures themselves. As previously discussed in our blogs, many research teams grapple with the challenges of multi-dimensional discovery in biologics R&D, necessitating the efficient integration and analysis of diverse data types – ranging from sequencing data to mass spectrometry data to flow cytometry data. These teams must strike a balance between the need for speed and accuracy in antibody discovery to find ways to advance antibody R&D with unified software and dataflows.
Additionally, there is an expanding array of potential antibody-based treatment options like bispecific or multispecific antibodies that show promise in addressing conditions like cancer, engineered antibody fragments, or even antibody-drug conjugates (ADCs). These require teams to blend the worlds of small molecule drugs and biologics, which require teams of biologists and chemists to collaborate and share data across scientific domains.[2]
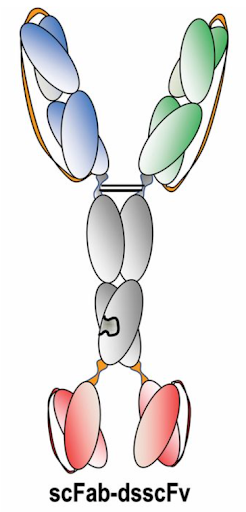
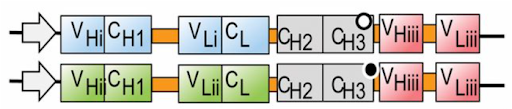
Figure 1: Designing novel multiformat antibodies—like bispecific antibodies that are built with “arms” that bind to distinct targets—creates unique challenges for researchers who must keep track of all the moving parts and explore how small design or component alterations impact activity. (Image credit: Weidle, U.H, Tiefenthaler, G., Weiss, G., et al. Cancer Genomics & Proteomics. 2013, Jan, 10 (1): 1-18.)
Three Must-Dos for Multiformat Antibody Design
As antibody formats get more complex, so does the process of tracking them. Take, for example, multispecific antibodies. These structures are often quite large and are connected in complicated ways, sometimes with multiple binding domains. Teams frequently mix and match different parts to explore the impact of small design changes. (Think of it as a “Frankenstein” approach to antibody design!) As teams iterate through these design changes and study their effects, they must thoroughly track an incredible amount of interconnected data, which is often easier said than done. An antibody discovery platform can make this daunting task easier by enabling teams to do three key things: manage the moving pieces, speak the same language, and track structural alterations. Let’s discuss.
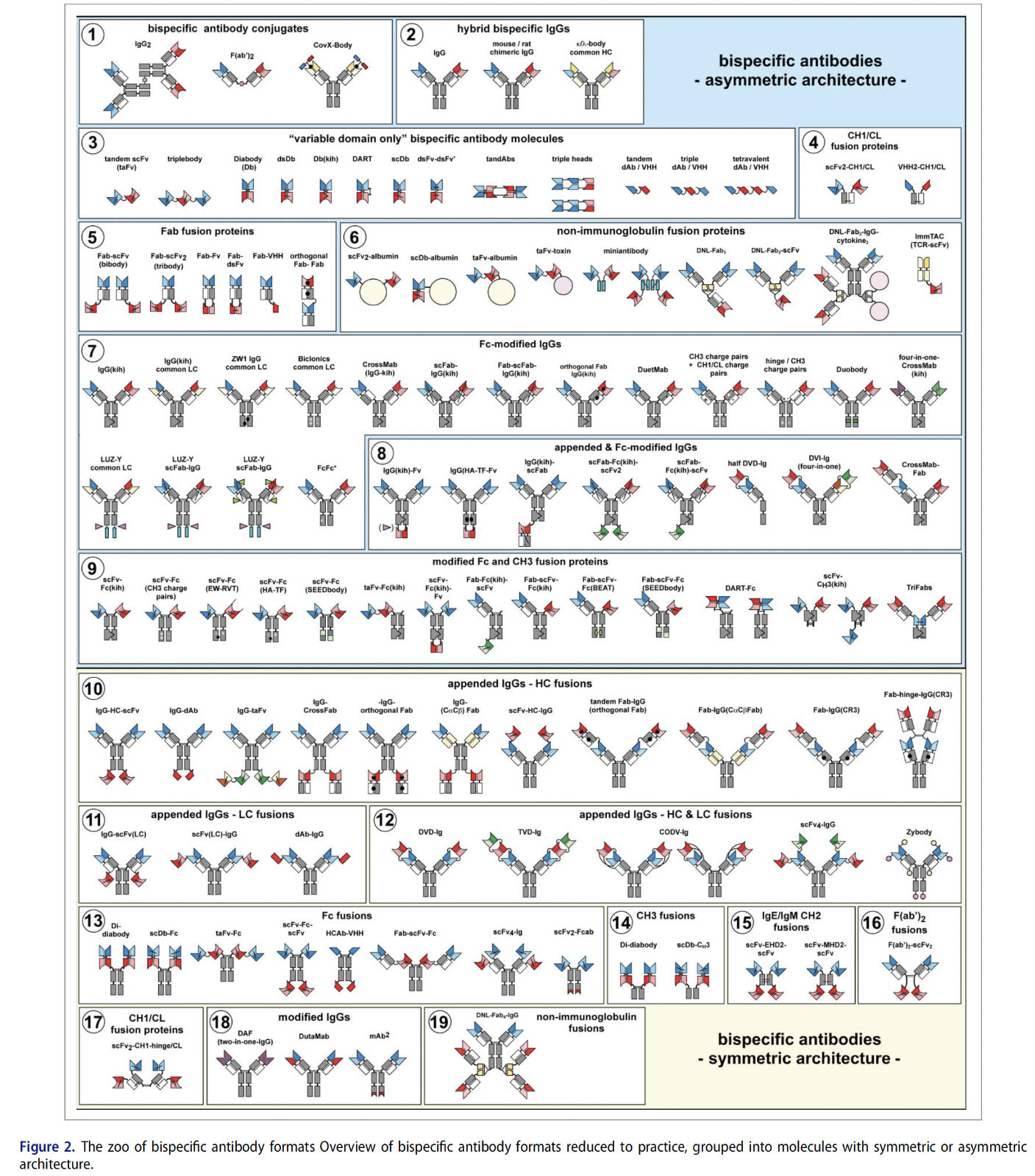
Figure 2: The zoo of bispecific antibody formats. Overview of bispecific antibody formats reduced to practice, grouped into molecules with symmetric or asymmetric architecture.
(Image credit: Brinkmann and Kontermann. MAbs. 2017 Feb-Mar; 9(2): 182–212) [4]
1. Manage Moving Pieces
Sequences. Variable regions. Linkers. Conjugates. All the various pieces of multiformat antibodies—and details regarding how they come together as a whole—need to be recorded and managed. Teams need to capture antibody design, assign unique identifiers to all components, record origin details, track modifications, analyze assembly, and assess activity. Unfortunately, many teams are still trying to manage all these moving parts with workaround solutions like massive spreadsheets. This quickly becomes unsustainable as researchers mix and match complex components through several design iterations, trying to find that magic combination.
2. Speak the Same Language
With so many moving parts at play, teams need to speak the same language in order to track projects and collaborate efficiently. As antibody structures become more complex, so does the task of naming them. Many teams struggle to establish or adopt consistency in naming conventions because their informatics systems don't offer an easy way to do so. But standardizing ontology is imperative. Teams need consistent ways to describe the antibody format type (e.g., bispecific or multispecific), record the source, identify the intended target, clarify the location and type of conjugates or radioisotopes, describe chemical modification, track product or project details, etc. Consistently capturing these details best positions teams to collaboratively design novel therapeutics; it is also a necessary precursor to machine-readiness. Ultimately, updating informatics solutions to use high-level code to describe structures in a way that both humans and computers can easily understand will be necessary for leveraging computer-aided design for AI antibody discovery.
3. Track Alterations
Teams often iterate through many design changes as they tweak not only the antibody components, but also how those components are interconnected, in order to optimize efficacy, specificity, and stability. Tracking these design changes, and the impact they have on performance, is a huge undertaking. On top of this, multiformat antibody design often requires structural alteration, such as with conjugation or chemical modification. These alterations can help overcome challenges with manufacturability, humanization, immunogenicity, and PK/PD/Tox. But, as many teams can attest to, collaborating across these types of chemically-modified biologics is challenging without a cross-discipline informatics solution that can thoroughly track both the intricate biological and chemical details of an entity.
Dotmatics Antibody Discovery Workflow Software
Dotmatics’ Antibody Discovery Workflow Solution accelerates antibody design by uniting all of the tools that teams need to analyze sequence data, design optimized candidates, and execute lab experiments on a comprehensive antibody discovery platform.
Multiformat Antibody Tracking
Realizing that the needs of antibody R&D teams are evolving as quickly as antibody formats themselves, our team at Dotmatics aims to make it easier for antibody R&D teams to manage all their moving pieces, standardize naming conventions, and record design alterations and their impact. With an off-the-shelf solution for managing all the complex details of multiformat antibody design, R&D teams can refocus their time on innovating and move from idea to reality quicker. Stay tuned for future updates about this exciting project!
References
The Antibody Society. Therapeutic monoclonal antibodies approved or in review in the EU or US. https://www.antibodysociety.org/resources/approved-antibodies/ (Accessed September 26, 2023)
Jin, S., Sun, Y., Liang, X. et al. Emerging new therapeutic antibody derivatives for cancer treatment. Sig Transduct Target Ther 7, 39 (2022). https://doi.org/10.1038/s41392-021-00868-x
Weidle, U.H, Tiefenthaler, G., Weiss, G., et al. Cancer Genomics & Proteomics. The Intriguing Options of Multispecific Antibody Formats for Treatment of Cancer. 2013, Jan, 10 (1): 1-18.
Brinkmann U, Kontermann RE. The making of bispecific antibodies. MAbs. 2017 Feb/Mar;9(2):182-212. doi: 10.1080/19420862.2016.1268307.