The Incredible Potential of CAR-T Therapy
When Carl June and colleagues first successfully reprogrammed patients’ T cells to identify and destroy cancer cells, it proved that patients’ own immune cells could be leveraged to treat complex disease, not just in theory, but in practice. Since that pivotal breakthrough, industry news has consistently highlighted the incredible potential for CAR-T cell therapy. The first CAR-T treatment was approved in 2017, and six different treatments are currently available for different types of blood cancers like leukemias and lymphomas.[1] June and colleagues, along with thousands of other experts around the globe, are now exploring ways to apply CAR-T cells to treat solid-tumor cancers, as well as diseases beyond cancer, including autoimmune diseases (such as lupus), heart failure, and more.[1] These researchers are devising a number of novel design approaches to overcome common limitations with first-generation CAR-T treatments, such antigen escape, off-tumor effects, tumor-infiltration challenges, and immunosuppression.[2-5].
CAR-T Hurdles—Safety and Cost
While next-generation CAR-T therapies offer hope for a wider range of patients, two major hurdles remain. First, the current paradigm of individualized treatment is extremely cost prohibitive. Second, adverse reactions, including cytokine storm, are still very common and patients need frequent monitoring. Current statistics show that as many as 75% to 100% of CAR-T patients experience cytokine storm.[6-7] These two factors combined make for an incredibly expensive treatment option. In fact, estimates for the total cost of CAR-T cell therapy—including developing personalized treatments and monitoring/managing adverse events—hover around $1 million.[8-9]
To overcome these obstacles, researchers are continuing to seek novel ways to make CAR-T therapies more tolerable, safe, universal, and cost effective. For example, experts at the University of Maryland School of Medicine are engineering CAR-T cells to target only cancer cells and ignore healthy blood cells, thereby reducing toxic side effects.[10] Researchers at University of Pennsylvania Perelman School of Medicine are turning to CRISPR base editing in an attempt to create more universal CAR-T therapies that can kill any type of blood cancer.[11] CRISPR is also being explored as a way to evolve CAR-T therapies from autologous patient-derived treatments to allogeneic donor-derived therapies.[12]
While these efforts are promising, a near-term fundamental paradigm shift is unlikely; in the meantime, we must optimize existing treatment paradigms while also making it easier for researchers to explore novel treatment approaches. Software solutions that streamline the assessment of CAR-T treatments using flow cytometry, as well as those that support collaborative CAR-T R&D, are key to this endeavor.
Flow Cytometry in CAR-T R&D and Treatment Assessment
Flow cytometry is a lab technique that uses lasers to conduct rapid, multiparametric evaluation of a high volume of cells within a stream of fluid, such as blood or disaggregated tissue samples. It can play a major role in both CAR-T R&D and manufacturing, as well as in the assessment of CAR-T treatment outcomes in a clinical setting. Flow cytometry can identify and characterize cell populations that help measure CAR-T cell presence and monitor disease status, as well as cell populations implicated in treatment response and adverse reactions, such as cytokine storm.
For example, flow cytometry can play a key role in assessing the composition of CAR-T products before they are released to patients. With flow cytometry, quality-control teams at pharmaceutical companies can test product samples using specific assays and biomarkers that help measure the number of singlets, live cells, T-cells, and most importantly, CAR+ cells, as shown in Figure 1. The composition of the product sample will help determine potency and inform manufacturing and dosing decisions; the speed at which the product analysis can be completed will have a major impact on how quickly potentially life-saving treatments can be released to patients.
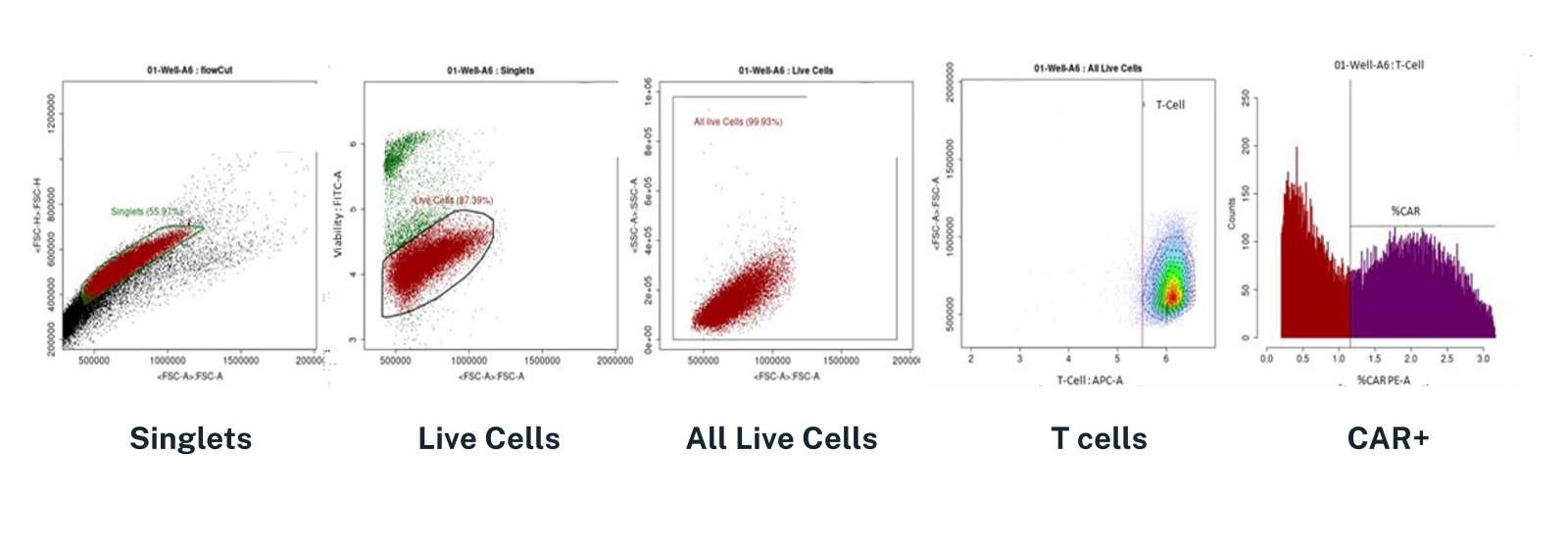
Figure 1: One of the many ways flow cytometry is used in the cell therapy space is to assess the composition of CAR-T products before they are released to patients, which can help determine potency and inform dosing decisions. The images here show results from an automated gating analysis of flow cytometry results, which quickly assessed the presence of singlets, live cells, T-cells, and CAR+ cells within a product sample. Streamlining this critical pre-product-release analysis is key to getting potentially life-saving products to critically-ill patients faster.
Unfortunately, the standard approach to analyzing flow cytometry results is inherently slow and labor-intensive. It is traditionally done through a process known as gating. In gating, highly-trained analysts identify cells of concern by manually drawing boxes around data points within scatter plots that are generated by the flow cytometry machine. The process takes around 30 minutes to 1.5 hours per sample in a clinical setting, which is unsustainable at any significant scale. The process is also highly variable due to analyst subjectivity. It is estimated that the subjectivity of manual human assessment can result in as much as 25% difference between analysts. And, while this cumbersome approach to turning raw flow cytometry data into actionable insights represents a glaring inefficiency in a research and manufacturing setting, the impact is even more concerning in a clinical setting, where it not only contributes to the cost burden of CAR-T therapy, but also poses a health risk to patients, such as when treatments are not properly QC’d or cannot be quickly released, or when poor treatment response or dangerous adverse events are not quickly flagged.
Automating Flow Cytometry Gating
Streamlining the assessment of flow cytometry results using advanced analysis and automation tools can have a significant impact. Automating flow cytometry gating, for example, can significantly speed up the notoriously inefficient gating process, saving both time and money. Automation can also lessen the reliance on specialty analysts and reduce the impact of human subjectivity that can sometimes lead to costly retesting. And, most importantly, by delivering reliable assessment results faster, automated gating provides the data and insights needed to more rapidly develop, optimize, and deliver potentially life-saving CAR-T therapies to critically-ill patients.
However, automating flow cytometry gating is no easy task; it is a balancing act between flexibility and specificity. On the one hand, the platform and algorithms used for automation must be flexible enough to support a team’s unique analysis pathways, including all pertinent assays and biomarkers. But on the other hand, they must also be able to account for the complex details needed to clearly define gating strategies and controls. The ultimate goal is an adaptive automation approach that provides results that are fast, valid, reliable, unbiased, and repeatable. The system should flag any files that don’t meet specific criteria so that they can receive assessment and reanalysis by an analyst as needed. And, ultimately, everything should work seamlessly at a high throughput scale, with results being returned in an easy-to-use format, such as graphics and data tables.
Dotmatics Solutions for CAR-T Treatment Assessment and CAR-T R&D
Dotmatics can help optimize the assessment of CAR-T treatments using flow cytometry, as well as support collaborative R&D for novel CAR-T therapies.
Dotmatics Flow Cytometry Analysis Software for CAR-T
Dotmatics reduces the burden of manual flow cytometry analysis by delivering powerful analysis and automation tools on one platform, helping analysts save time and make sense of data faster. Dotmatics flow cytometry solutions, including OMIQ and FCS Express, help R&D researchers and clinical analysts:
Connect with flow cytometers to directly pull raw instrument data and metadata, as well as monitor instrument performance.
Identify cells areas of interest with gating and automated gating tools.
Scrutinize cell subpopulations of interest using specialty analysis, statistics, graphing, and reporting programs.
Leverage machine learning and analytical pipelines to take analyses to the next level.
Dotmatics CAR-T R&D Workflow
Dotmatics CAR-T workflow unites all the tools researchers need to design cell-based therapies like CAR-T. These include leading molecular biology and bioinformatics tools like SnapGene and Geneious, as well as a collection capabilities that help teams streamline collaborative scientific exploration, such as a configurable ELNs, assay and sample management tools, an entity registration system, and visualization and statistics tools for analyzing data and sharing insights.
Learn More
Contact us today to learn how Dotmatics can optimize your CAR-T workflows.
References
Raeke, M. The Boundless Potential of CAR T Cell Therapy, From Cancer to Chronic and Common Diseases: A Q&A with Carl June. Penn Medicine News. August 22, 2023.
Sterner, R.C., Sterner, R.M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021). https://doi.org/10.1038/s41408-021-00459-7
Tomasik, J., Jasinski, M. and Basak, G.W. Next generations of CAR-T cells - new therapeutic opportunities in hematology? Front. Immunol., 13 (2022).| https://doi.org/10.3389/fimmu.2022.1034707
Bailey SR, Berger TR, Graham C, Larson RC, Maus MV. Four challenges to CAR T cells breaking the glass ceiling. European Journal of Immunology. 2022 Dec:e2250039. DOI: 10.1002/eji.202250039.
Yan, T., Zhu, L. & Chen, J. Current advances and challenges in CAR T-Cell therapy for solid tumors: tumor-associated antigens and the tumor microenvironment. Exp Hematol Oncol 12, 14 (2023). https://doi.org/10.1186/s40164-023-00373-7
CAR-T Therapy and the Cytokine Storm. American College of Emergency Physicians - Critical Care Medicine.
Jensen, M. and Gardner, R. A Novel Method to Prevent CAR T-cell-induced Cytokine Storm. Seattle Children’s Hospital. Website. (Accessed October 17, 2023).
Szabo, L. Cascade of Costs Could Push New Gene Therapy Above $1 Million Per Patient. KFF News. October 17, 2017.
Robinson, K.M. Navigating the Financial Aspects of CAR T-Cell Therapy. WebMD. January 24, 2023.
Kotz, D. New Innovation in CAR T-Cells Paves Way for Less Toxic Therapy Against Multiple Myeloma. University of Maryland School of Medicine - News. July 19, 2023.
Phillips, C. Researchers Develop a Potential “Universal” CAR T-Cell Therapy for Blood Cancers. National Cancer Institute: Cancer Currents Blog. September 27, 2023.
Henderson, H. CRISPR Clinical Trials: A 2023 Update. Innovative Genomics Institute. March 17, 2023.