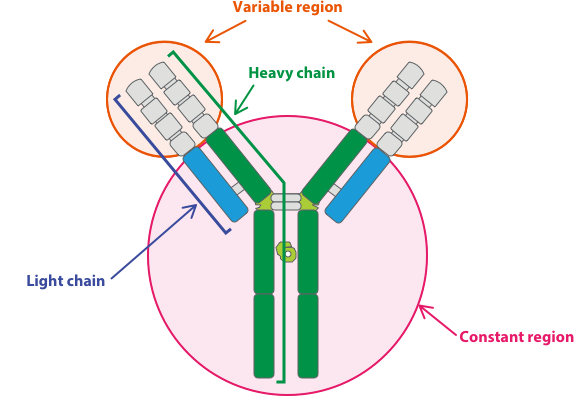
Biologics researchers face countless choices in early discovery that can greatly impact their candidate selection and downstream R&D efforts. Unfortunately, for many teams, quickly making well-informed decisions feels next-to-impossible because their data, workflows, and teams aren’t working in unison. At BioIT-World Expo and Conference, Christian Olsen, Dotmatics’ Associate VP and Industry Principal for Biologics, discussed how Dotmatics’ flexible end-to-end R&D platform changes this paradigm, uniting all the data and tools researchers need to quickly uncover promising preclinical biologics candidates and significantly reduce time spent managing data. We review some highlights from his presentation below.
Multi-Dimensional Discovery in Biologics R&D
Biologics R&D teams are pursuing complex treatments, such as antibodies, to address complex disease. Making breakthroughs often hinges on building new knowledge by combining and recombining different types of research data. At Dotmatics, we refer to this as “research composability.” Biologics teams often leverage many different types of tools and analyses to look at things through multiple different lenses, including:
sequencing (e.g., B-cell, T-cell repertoire sequencing)
mass spectrometry and high-performance liquid chromatography (primary structures, post translational modifications, glycan structures)
flow cytometry (immune cell characterization)
While very specific knowledge can be gleaned by looking through these different lenses, that knowledge is only partially useful when viewed in isolation. Combining the insights attained through each lens is the best way to create a complete candidate profile.
Obstacles to Multidimensional Discovery in Biologics R&D
However, attaining a complete candidate profile can be incredibly difficult. For most teams, this stems from one key struggle—data. In particular, issues often arise due to data diversity and data silos, as well as from the difficulty of passing data from task-to-task and team-to-team.
Data Diversity and Data Silos
Biologics research is typically quite diverse. Companies often have different research groups, who are producing different types of results, using different types of speciality technologies, at different times throughout the R&D discovery process. As shown in Figure 1, this can lead to an incredibly complex and interconnected data web.
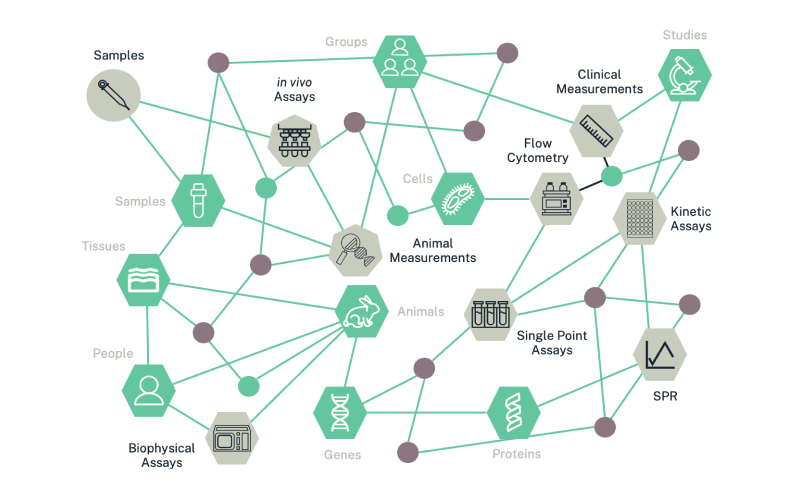
Figure 1: The web of data resulting from diverse biologics discovery efforts is exceptionally challenging to manage, relate and decipher, leading to R&D inefficiencies that waste time and money.
Not only is this web of data difficult to untangle, but it often causes teams to struggle with:
Huge volumes and variety of complex data
Disconnected data silos (e.g., protein, assay, flow, sequence, image, animal, antibody, patient, etc).
Inaccurate, outdated, duplicated, inaccessible, or lost data
Incomplete data (e.g., antigen-unlabeled data with no recorded connection between antibody and antigen, limited antigen-specificity data to link factors like age, health, or geographic region to help teams better understand adaptive immunity).
Fractured infrastructure and incompatible speciality tools (e.g., tools for high-throughput antibody sequence analysis, single-cell analysis, and glycoprotein analysis don’t feed data into a interoperable master data source)
Disjointed (sometime redundant) workflows with no easy dataflow from task-to-task or team-to-team
By some estimates, up to 30% of annual revenue can be lost from inefficiencies resulting from incorrect or siloed data.
Data Transfer Between Tasks and Teams
Data silos also significantly impact how easy it is for researchers to switch from one task to another, or share data with other research groups or CRO collaborators. When data are locked in disconnected silos, workflow and productivity suffer because researchers can’t easily get the data they need when they need it. The onerous process of switching tasks and transferring data not only wastes time, but also interrupts concentration and risks error. But, task switching is an essential need in multi-dimensional discovery, where researchers often shift between different types of experiments or data analyses to identify patterns and make connections that may help them gain new insights or perspectives.
Make Better Decisions, Faster
With better dataflow and workflow, teams can quickly create complete candidate profiles that let them make better decisions faster. But achieving this is only possible with a flexible end-to-end R&D platform like Dotmatics Platform, which provides:
A single source of scientific truth with FAIR data that are easy to find and share between applications and teams
A rich collection of speciality applications that biologics researchers need to scrutinize candidates
Ability to overlay many types of complex data (e.g., sequence, flow, mass spec) to construct a rich candidate profiles and quickly make data-driven decisions
Workflow efficiency and productivity tools to refocus researchers’ time from data handling back to scientific exploration
Example: Improved Dataflow and Workflow in Antibody Sequence Discovery
Only Dotmatics offers the scientific depth and true enterprise architecture needed to let scientists dive into granular detail and tie back their discoveries to the bigger picture. As shown in Figure 2, Dotmatics offers a complete Antibody Discovery Solution that supports every step of the Make-Test-Decide innovation cycle. Speciality functionality available within the solution includes:
Make - SnapGene, Geneious Prime
Test - Protein Metrics, Prism, OMIQ, FCS Express
Decide - Geneious Biologics
These speciality capabilities can be coupled with Dotmatics’ core data and productivity capabilities, including ELN, entity registration, sample and task management, scientific search, data visualization and analytics, assay screening and management, instrument integration, and third-party openness. The combined solution unites the dataflows and workflows needed to analyze candidates through multiple innovation-cycle iterations. With each trip around the innovation cycle, the pool of targets decreases and the value of the data increases. Having powerful tools to capture, share, aggregate, and analyze that data is essential to making the right decisions, faster. With Dotmatics, the web of biologics discovery data becomes unified, connected, organized, and standardized in an open and flexible repository, as shown in Figure 2.
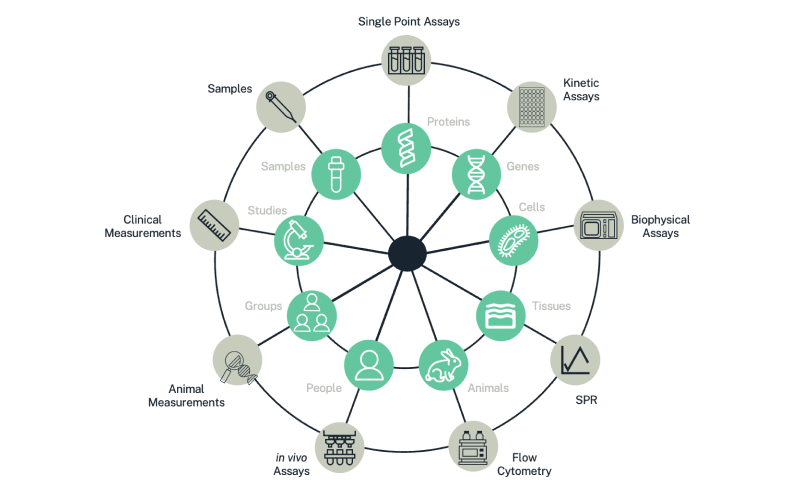
Figure 2: Dotmatics helps untangle the web of discovery data, optimizing workflows and dataflows and helping researchers make connections that will help drive innovation.
With Dotmatics, R&D teams can achieve up to a:
70% reduction in data integration and analysis, leaving more time for conducting experiments
50% reduction in documentation creation, increasing time for collaboration.
Next Steps
Request a product demonstration with a product expert to see for yourself how The Dotmatics Biology Solution for Biologics Drug Discovery can help your team make the right decisions, faster.